
GERD (gastroesophageal reflux disease) remains extremely common, affecting an estimated 40% of Americans on at least a weekly basis, despite widespread use of acid-suppressing therapies such as antacids, H2 blockers, and proton-pump inhibitors (PPIs). I have written previously (1) about my concerns regarding long-term use of these medications, particularly the decreased absorption of critical minerals and other nutrients caused by lack of adequate stomach acid. In this article I would like to highlight some evidence-based non-pharmacologic treatment options that improve normal function of the esophagogastric junction and may therefore decrease reflux occurrence and symptoms and ultimately the need for PPIs.
Over the past 10 years or so there has been increasing recognition within the medical literature that the diagnosis of GERD actually consists of a spectrum of conditions. (2, 3, 4, 5) The classic symptom picture of erosive esophagitis (with reflux of stomach acid into the esophagus that causes tissue damage over time) appears to describe a minority of patients with chronic heartburn, while non-erosive reflux (NERD) subtypes account for 50-85% of cases. Most NERD patients are only identified when their symptoms fail to improve on empiric PPI therapy.
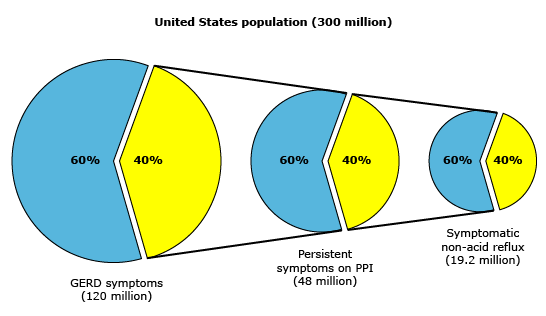
“True” NERD involves abnormal acid exposure within the lower esophagus but no mucosal tissue changes (such as erosions or ulcerations) are visible on upper endoscopy. The other subtypes of NERD are designated as esophageal hypersensitivity and “functional” heartburn; these do not involve abnormal acid exposure but likely involve increased chemical and mechanical sensitivity within the esophagus for a variety of reasons including altered mucosal integrity and/or immunity, increased nerve density, and central sensitization. (6, 7, 8, 9, 10, 11, 12)
Proper workup and diagnosis of reflux symptoms is therefore essential for individualized treatment. At minimum, I prefer to have all patients with GERD undergo an upper endoscopy procedure. EGD (esophagogastroduodenoscopy) examines the health of the mucosa in the esophagus, stomach, and duodenum. This technique identifies erosive esophagitis when it is present (so we can use acid-suppressing medications when they are most needed) and allows biopsy of tissue samples to assess for conditions that can mimic or exacerbate reflux such as eosinophilic esophagitis, atrophic gastritis, H. pylori infection, etc.
The best test for diagnosing the subtypes of NERD seems to be combined impedance and pH testing, which measures the frequency and duration of reflux episodes as well as the acidity of the refluxate fluid. Currently this technique is used mostly in research studies but I expect it will become more common in clinical practice over the next few years.
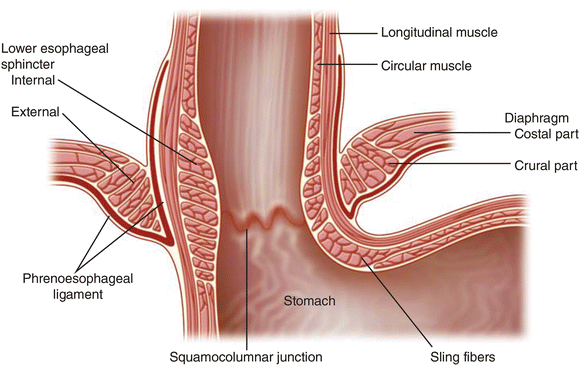
The normal anatomy at the junction of the esophagus and stomach is intended to prevent reflux. The stomach should sit in the abdomen below the diaphragm, positioned so the lower esophageal sphincter (LES) muscle is reinforced by the crural section of the diaphragm. Resting pressure within the LES is normally maintained at 10-30 mmHg, with fluctuations throughout the day (lowest after meals and highest at night). Pressure at the esophagogastric junction (EGJ) also varies with breathing; during inhalation the contraction of the crural diaphragm increases EGJ pressure up to 3-4x. (13)
Gastroesophageal reflux occurs when either or both parts of this muscle barrier become weakened, thereby allowing frequent transient LES relaxations (TLESRs). This phenomenon is a normal venting mechanism (i.e. to allow belching) when the stomach is distended by food or gas. However, in GERD patients the baseline LES pressure tends to be much lower than normal, often below 10 mmHg. (14)
Not all the reasons for EGJ hypotonia are well understood, but parasympathetic nervous system dysfunction appears to be a major underlying cause. The crural diaphragm and much of the esophagus are innervated by the vagus nerve in addition to spinal nerves. (15) Since the parasympathetic nervous system is responsible for “rest and digest” functions, it makes sense that chronic activation of the sympathetic nervous system (“fight or flight”) would have numerous negative impacts on digestive function:
- altered gastrointestinal motility;
- increased visceral perception;
- decreased gastrointestinal secretion;
- increased esophageal and intestinal permeability; and
- decreased regenerative capacity of gastrointestinal mucosa and mucosal blood flow. (16, 17)
Chronic stress has been shown to aggravate GERD symptoms due to inhibition of the LES, increased esophageal sensitivity to acid reflux (18), impaired ability of the stomach to accommodate and mechanically digest food, and delayed gastric emptying. (19)
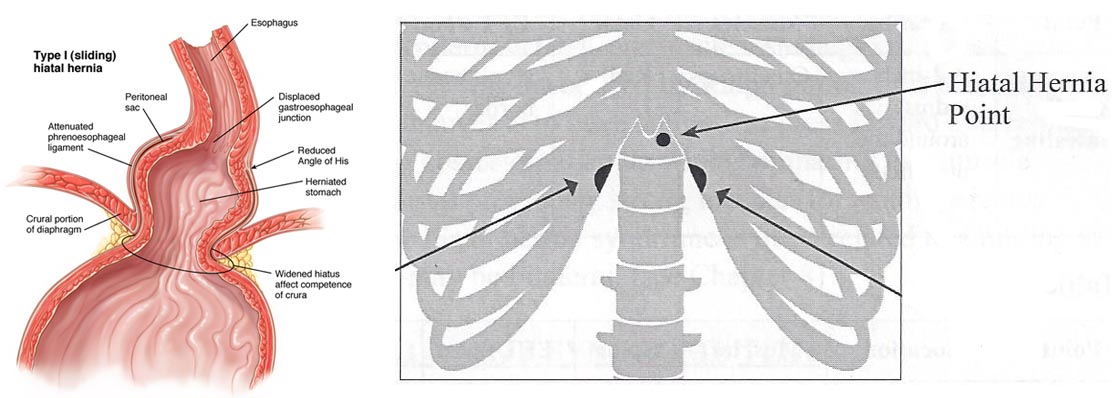
Weakness of the crura combined with chronic or episodic increases in intra-abdominal pressure (such as from obesity, pregnancy, exertion while holding the breath, weightlifting, jumping on a trampoline, etc.) allow stretching of the fascial attachments between the esophagus and diaphragm and eventually the protrusion of the stomach through the hiatus (opening) of the diaphragm, creating a hiatal hernia. When this occurs, the diaphragm no longer acts as part of the anatomical reflux barrier but rather contributes to reflux as it compresses the upper part of the stomach. A “sliding” type of hiatal hernia is most common and may occur in 40% of the general population. (20) Hiatal hernia can often be identified by tenderness at the Riddler hiatal hernia reflex point located just below and just to the left of the xyphoid process at the base of the sternum. (21) Although some hernias may be identified on EGD, the best test to detect a hiatal hernia remains a barium swallow x-ray. (22) A large number of hiatal hernias may never be diagnosed until found incidentally during abdominal surgery – as many as 43% in one case series. (23)
From the above discussion, we see that an optimal treatment approach for GERD would attempt to improve the normal structure and function of the esophagogastric junction. The plan would:
- soothe, protect, and stimulate regeneration of the esophageal mucosa;
- improve the muscle tone of the LES and crural diaphragm and promote downward GI motility; and
- identify and address dietary and lifestyle factors that aggravate reflux.
(Disclaimer: please note that none of these recommendations should be considered a substitute for your individualized medical evaluation and treatment).
Part 1: Soothe, protect, and stimulate
- Acid-suppressing medications as needed for short-term use (ideally ≤ 2 weeks), particularly when erosive esophagitis or severe hypersensitivity are present
- Demulcent herbs to soothe irritated mucosa (24)
- Hyaluronic acid and chondroitin to accelerate tissue healing (25, 26)
- Melatonin to stimulate mucosal blood flow (27)
- Zinc carnosine to support mucosal cell repair (28, 29)
- Probiotics to support mucosal immunity and parasympathetic nervous system tone (30, 31)
Part 2: Improve EGJ pressure and promote downward GI motility / energy flow
- Visceral manipulation of the stomach can sometimes be used to reduce a hiatal hernia. (32) Unfortunately, this technique may not be permanent for patients with a sliding hiatal hernia but it may become more useful over time.
- Chiropractic evaluation and treatment often reveals muscle tightness and bony restrictions in the lower thoracic spine. Thoracolumbar kyphosis and scoliosis can negatively impact diaphragm function and increase intra-abdominal pressure with compression of the esophagus and stomach. (33)
- Myofascial release of the diaphragm may be useful, especially following chiropractic care. (34)
- Acupuncture has been shown to promote downward/forward motility through the digestive tract in animals and humans. (35, 36)
- In Chinese medicine, GERD is considered a manifestation of qi that has become stagnant; this is often associated with holding things in on an emotional level. Counseling and journaling may be useful ways of learning to “let it go, let it go!” (37)
- Deep breathing exercises should be performed to engage the diaphragm. Slow abdominal breathing improves parasympathetic nervous system tone (38) and decreases esophageal sensitivity. (39) There is also exciting research which suggests that the diaphragm can be strengthened like any other muscle and therefore breathing training may improve GERD symptoms by increasing muscle tone in the LES and crural diaphragm. (40) Several studies have used a Threshold IMT device for progressive resistance breathing training (like lifting weights for your diaphragm). (41, 42)
- Supine bilateral leg raises have been shown to increase LES pressure; (43) they also build core strength.
Part 3: Address diet and lifestyle habits
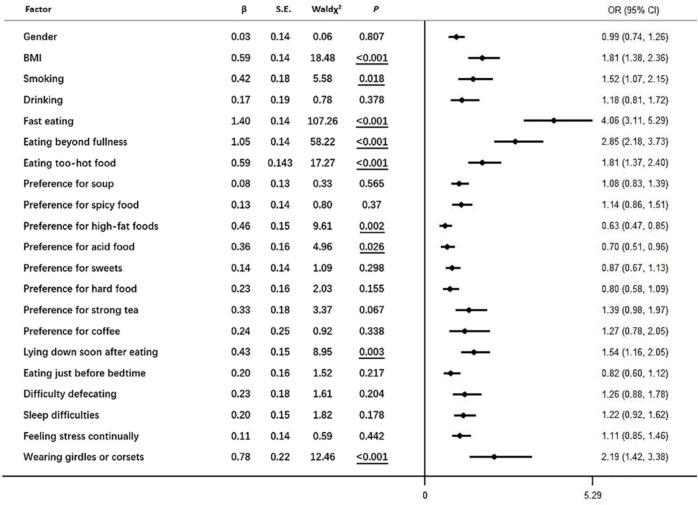
A study by Yuan et al. determined the major lifestyle risk factors among 699 patients with GERD. These are tabulated here both by significance (P values < 0.05, which are underlined) and likelihood (odds ratio > 1.0): eating too quickly (< 10 minutes per meal and chewing < 10 times per bite) (OR 4.06), eating beyond fullness (2.85), wearing restrictive clothing (eg. corset, girdle) (2.19), obesity with BMI > 24 (1.81), eating very hot foods (> 60 °C) (1.81), lying down after eating (1.54), smoking (at least one cigarette per day) (1.52). (44)
The 699 patients were followed for 6 months. All participants were treated with PPI therapy (20 mg omeprazole) and counseled on 20 lifestyle factors. Of the 464 patients who followed all lifestyle recommendations, 56.9% had substantial improvement and 41.4% had moderate improvement; only 1.7% had no improvement. (By contrast, of the 235 patients who did not follow lifestyle recommendations, 26.4% had substantial improvement, 59.6% had moderate improvement, and 14.0% had no improvement).
From this list we can easily observe that relatively simple changes may have a big impact. General dietary recommendations as supported by the most recent research literature are as follows:
- Eat slowly and chew food thoroughly. This is probably the single most important modifiable risk factor for most patients. Eating too quickly encourages overeating which distends the stomach (increasing TLESRs and reflux episodes) and slows digestion and therefore delays gastric emptying. Swallowing large mouthfuls of rough, half-chewed food or very hot food may damage mucous membranes. Habitual rapid food intake may eventually cause ineffective esophageal motility; chewing gum may strengthen weakened esophageal muscles. (45)
- Limit snacking between meals; after every feeding, gastric acid is secreted and the acid pocket reforms. (46) Eating only two meals per day has been shown to decrease symptoms in GERD patients with reflux esophagitis within 2 weeks. (47)
- Avoid drinking more than a few ounces of fluids during mealtime (30 minutes before food and 60-120 minutes after) to avoid over-filling the stomach and diluting stomach acid. (48)
- Limit coffee, tea, and soda (or similar carbonated beverages) which are associated with increased reflux; replacing 2 servings of these beverages with water can decrease GERD symptoms. (49)
- The best food choices will vary person to person. Acidic and spicy foods tend to be most problematic due to direct irritation of the mucosa; alcohol, coffee, chocolate, and mint decrease LES muscle tone. (50) Food intolerance testing or an elimination diet may be helpful for identifying food triggers. Almost all patients in one retrospective study were intolerant to at least 5 food items; milk, lettuce, coffee, yeast, pork, tuna, sole, rice, asparagus, and eggs were most common. (51)
- Fatty foods can trigger reflux in some patients. (52) However, high carbohydrate intake can also contribute. A study by Pointer et al. of dietary habits among 144 overweight women with GERD revealed that sugar intake was significantly associated with reflux symptoms, with the odds increasing by 13% for every additional teaspoon (4.2 g) of sugars consumed. Over the 16 weeks of the study, carbohydrate intake was decreased from 45 to 35% of total calories (1/2 complex carbs and 1/2 simple carbs) and fat intake was increased from 38 to 48% of total calories (1/3 saturated, 1/3 monounsaturated, 1/3 polyunsaturated), while keeping protein intake the same at 17% of total calories. All of the women with GERD had resolution of their symptoms and were able to discontinue PPI medications within 10 weeks! (53) Other studies have shown that very-low carbohydrate diets (< 20 grams per day) also reduce distal esophageal acid exposure and improve heartburn symptoms. (54, 55) GERD triggered by carbohydrate intake is typically related to the effects of bacterial fermentation of undigested starches in the large intestine; studies by Piche et al. have shown that consumption of fermentable starches and the presence of short-chain fatty acids in the colon dramatically decreases LES pressure by 10 mmHg, increasing the number of TLESRs and reflux episodes. (56, 57) Anywhere from 2-20% of dietary starches may remain undigested in healthy people; malabsorption conditions may increase this percentage, and dysbiosis conditions (such as SIBO) may increase the rate of fermentation within the intestines. Interestingly, increased intake of non-fermentable fiber such as psyllium 5 grams 2-3x per day can decrease GERD symptoms comparable to omeprazole. (58, 59)
- Weight loss resulting in a decrease of ≥ 2 kg/m2 BMI and ≥ 2 inches waist circumference improves GERD sxs in obese patients with baseline BMI > 25 by decreasing intra-abdominal pressure. (60)
- https://willametteintegrative.com/2014/10/23/purple-pill-not-enough-for-gerd/
- Mahoney LB, Rosen R. The spectrum of reflux phenotypes. Gastroenterol Hepatol (NY) 2019;15(12):646-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6935024/
- Herschcovici T, Fass R. Nonerosive reflux disease (NERD) – an update. J Neurogastroenterol Motil 2010;16(1):8-21. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879816/pdf/jnm-16-8.pdf
- Chen CL, Hsu PI. Current advances in the diagnosis and treatment of nonerosive reflux disease. Gastroenterology Research and Practice 2013;(2013):653989. http://www.hindawi.com/journals/grp/2013/653989/
- Tutuian R et al. Non-acid reflux: clinical manifestations, diagnosis, and management. UpToDate 2018. https://www.uptodate.com/contents/non-acid-reflux-clinical-manifestations-diagnosis-and-management
- Frazzoni M et al. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive esophagus from functional heartburn. J Gastroenterol 2017;52(4):444-51.
- Kandulski A et al. Histomorphological differentiation of non-erosive reflux disease and functional heartburn in patients with PPI-refractory heartburn. Aliment Pharmacol Ther 2013;38(60:643-51. https://onlinelibrary.wiley.com/doi/full/10.1111/apt.12428
- Woodland P et al. Distinct afferent innervation patterns within the human proximal and distal esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 2015;308(6). https://journals.physiology.org/doi/full/10.1152/ajpgi.00175.2014
- Woodland P et al. Superficial esophageal mucosal afferent nerves may contribute to reflux hypersensitivity in nonerosive reflux disease. Gastroenterol 2017;153(5):1230-9.
- Hungin APS et al. Revisiting Montreal: new insights into symptoms and their causes, and implications for the future of GERD. Am J Gastroenterol 2019;114(3):414-21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6434899/
- Gabbard S et al. Functional heartburn: an underrecognized cause of PPI-refractory symptoms. Cleve Clin J Med 2019;86(12):799-806. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/issues/articles/799.pdf
- Yang M et al. Quantitative assessment and characterization of visceral hyperalgesia evoked by esophageal balloon distention and acid perfusion in patients with function heartburn, nonerosive reflux disease, and erosive esophagitis. Clin J Pain 2010;26(4):326-31.
- Sun XH et al. Roles of diaphragmatic crural barrier and esophageal body clearance in patients with gastroesophageal reflux disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002;24(3):289-93.
- Lieberman DA. Medical therapy for chronic reflux esophagitis. Long-term follow-up. Arch Intern Med 1987;147(10):1717-20.
- Young RL. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology 2010;138(3):1091-101. https://www.gastrojournal.org/article/S0016-5085(09)01511-X/fulltext
- Konturek PC et al. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 2011;62(6):591-9. http://www.jpp.krakow.pl/journal/archive/12_11/pdf/591_12_11_article.pdf
- Farré R et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intracellular spaces. Gut 2007;56(9):1191-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1954970/'
- Broers C et al. The effect of intravenous corticotropin-releasing hormone administration on esophageal sensitivity and motility in health. Am J Physiol Gastrointest Liver Physiol 2017;312(5):G526-34.'
- Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47(6):861-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1728136/pdf/v047p00861.pdf
- http://www.merckmanuals.com/professional/gastrointestinal_disorders/esophageal_and_swallowing_disorders/ hiatus_hernia.html
- Sandberg-Lewis S. Functional Gastroenterology. Portland, OR: NCNM Press, 2009.
- Sfara A, Dumitrascu DL. The management of hiatal hernia: an update on diagnosis and treatment. Med Pharm Rep 2019;92(4):321-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6853045/
- Hutopila I et al. Hiatal hernia is more frequent than expected in bariatric patients. Intraoperative findings during laparoscopic sleeve gastrectomy. Chirurgia 2019;114:779-89. http://revistachirurgia.ro/pdfs/2019-6-779.pdf
- Panahi Y et al. Efficacy and safety of aloe vera syrup for the treatment of gastroesophageal reflux disease: a pilot randomized positive-controlled trial. J Tradit Chin Med 2015;35(6):632-6. http://www.journaltcm.com/modules/Journal/contents/stories/156/4.pdf
- Chmielecka-Rutkowska J et al. The role of oral hyaluronic acid preparation and chondroitin sulfate in the treatment of patients with laryngopharyngeal reflux. Polish J Otolaryngology 2019;73(6). https://otolaryngologypl.com/resources/html/article/details?id=194903&language=en
- Palmieri B et al. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci 2013;17(24):3272-8. http://www.europeanreview.org/wp/wp-content/uploads/3272-3278.pdf
- Konturek SJ et al. Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J Physiol Pharmacol 2006;57 Suppl 5:51-66.
- Ozbayoglu A et a. Effect of polaprezinc on experimental corrosive esophageal burns in rats. Dis Esophagus 2017;30(11):1-6.
- Zhang Q, Feng L. Protective effect of polaprezinc on acute gastric mucosal injury in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019;44(1):22-7. http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/20190122.pdf
- Cheng J, Ouwehand AC. Gastroesophageal reflux disease and probiotics: a systematic review. Nutrients 2020;12(1).
- Bravo JA et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011; 108(38):16050-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3179073/
- Dombrowski A, Imre K, Sandberg-Lewis S, Zwickey H. Treatment of gastrointestinal symptoms and mood disorder with physical medicine and supplementation: a case report. Integr Med (Encinitas) 2018;17(3):53-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6396765/
- Ohba T et al. Prevalence and key radiographic spinal malalignment parameters that influence the risk for gastroesophageal reflux disease in patients treated surgically for adult spinal deformity. BMC Gastroenterol 2018;18:8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5763649/
- Martínez-Hurtado I et al. Effects of diaphragmatic myofascial release on gastroesophageal reflux disease: a preliminary randomized controlled trial. Sci Rep 2019;9:7273. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6513998/
- Dickman R et al. Clinical trial: acupuncture vs doubling the proton pump inhibitor dose in refractory heartburn. Aliment Pharm Ther 2007;26(10):1333-44. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2036.2007.03520.x
- Han G et al. Electroacupuncture to treat gastroesophageal reflux disease: study protocol for a randomized controlled trial. Trials 2016;17:246. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869308/
- https://www.tcmworld.org/the-body-never-lies-acid-reflux/
- Kocjan J et al. Network of breathing. Multifunctional role of the diaphragm: a review. Adv Respir Med 2017;85(4):224-32. https://journals.viamedica.pl/advances_in_respiratory_medicine/article/view/ARM.2017.0037/41543
- Botha C et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 2015;64(4):611-17.
- Casale M et al. Breathing training on lower esophageal sphincter as a complementary treatment of gastroesophageal reflux disease (GERD): a systematic review. Eur Rev Med Pharmacol Sci 2016;20:4547-52. https://www.europeanreview.org/wp/wp-content/uploads/4547-4552-Breathing-training-on-lower-esophageal-sphincter.pdf
- Nobre e Souza MÂ. Inspiratory muscle training improves antireflux barrier in GERD patients. Am J Physiol Gastrointest Liver Physiol 2013;305(11):G862-7. https://journals.physiology.org/doi/full/10.1152/ajpgi.00054.2013
- Carvalho de Miranda Chaves R et al. Respiratory physiotherapy can increase lower esophageal sphincter pressure in GERD patients. Respir Med 2012;106(12):1794-9.
- Bitnar P et al. Leg raise increases pressure in lower and upper esophageal sphincter among patients with gastroesophageal reflux disease. J Bodyw Mov Ther 2016;20(3):518-24.
- Yuan LZ et al. Lifestyle intervention for gastroesophageal reflux disease: a national multicenter survey of lifestyle factor effects on gastroesophageal reflux disease in China. Therap Adv Gastroenterol 2019;12:1756284819877788. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6764031/
- Li KL et al. Habitual rapid food intake and ineffective esophageal motility. World J Gastroenterol 2013;19(14):2270-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3627893/
- Fiorentino E. The consumption of snacks and soft drinks between meals may contribute to the development and to the persistence of gastroesophageal reflux disease. Med Hypotheses 2019;125:84-8.
- Randhawa MA et al. An old dietary regimen as a new lifestyle change for gastro esophageal reflux disease: a pilot study. Pak J Pharm Sci 2015;28(5):1583-6.
- Kusano M et al. Postprandial water intake inhibits gastric antral motility with increase of cholecystokinin in humans. Scand J Gastroenterol 2005;40(10):1176-81.
- Mehta RS et al. Association between beverage intake and incidence of gastroesophageal reflux symptoms: beverages and GER symptoms. Clin Gastroenterol Hepatol 2019. [epub ahead of print]. https://www.ncbi.nlm.nih.gov/pubmed/31786327
- Newberry C, Lynch K. The role of diet in the development and management of gastroesophageal reflux disease: why we feel the burn. J Thorac Dis 2019;11(Suppl 12):S1594-1601. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6702398/
- Caselli M et al. Pattern of food intolerance in patients with gastroesophageal reflux symptoms. Minerva Med 2017;108(6):496-501.
- Clavé P et al. Endogenous cholecystokinin enhances postprandial gastroesophageal reflux in humans through extrasphincteric receptors. Gastroenterology 1998;115(3):597-604.
- Pointer SD et al. Dietary carbohydrate intake, insulin resistance, and gastroesophageal reflux disease (GERD): a pilot study in European- and African-American obese women. Aliment Pharmacol Ther 2016;44(9):976-88. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5048546/
- Austin GL et al. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci 2006;51(8):1307-12.
- Langella C et al. New food approaches to reduce and/or eliminate increased gastric acidity related to gastroesophageal pathologies. Nutrition 2018;54:26-32.
- Piche T et al. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology 2003;124:894-902. https://www.gastrojournal.org/article/S0016-5085(03)00073-8/pdf
- Piche T et al. Modulation by colonic fermentation of LES function in humans. Am J Physiol Gastrointest Liver Physiol 2000;278(4):G578-84 https://www.physiology.org/doi/pdf/10.1152/ajpgi.2000.278.4.G578
- Morozov S et al. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol 2018;24(21):2291-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5989243/
- Hosseini M et al. Comparing the effect of psyllium seed on gastroesophageal reflux disease with oral omeprazole in patients with functional constipation. J Evid Based Integr Med 2018;23:2515690X18763294. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5888803/
- Park SK et al. Weight loss and waist reduction is associated with improvement in gastroesophageal disease reflux symptoms: a longitudinal study of 15,295 subjects undergoing health checkups. Neurogastroenterol Motil 2017;29(5).
















5 comments
This is very informative, thank you! I have to honestly say, I suffered from really bad GERD, taking OTC medications for years that I later found were leaching my body of D and B vitamins, and on top of that taking PecidAC and chewing antacids and STILL suffering half the time. When I started the AIP diet within about 3-4 days (and I had stopped taking the OTC drugs when I started) my GERD went away. That’s been one of the most immediate and dramatic benefits since I began! It’s been wonderful.
Kerstin, we are so happy to hear this!
Wow! Thanks for sharing your experience Kerstin!
I have unfortunately the opposite experience. After 6 weeks on AIP I have sort of heartburn, specially during night time. Any idea why this is happening?
Hi Hanne! Sorry to hear – your best bet is to seek out the help of an experienced coach to help troubleshoot with you. You can find a listing of providers here: http://aipcertified.com/coach-directory