
Over the last several weeks I have pondered greatly the best way to articulate thoughts and ideas I have had on race authentically and through the lens of a medical provider who is still in the work of educating and discovering the impact of systemic oppression on health. My intention in this article is to offer a clear discussion into the truth behind race and genetics, the potential enduring health effects of systemic oppression, and the hopeful way forward to heal and relieve suffering.
Defining Race
As I have continued to think about the words “race” and “racism”, I have repeatedly stumbled trying to make meaning from their intended use. Race, in my view, reflects the universal human “race” or human species Homo sapiens. Race from this lens should therefore be the unifier of humanity, not the divider. The term “race,” as it is more commonly used now, appears to reflect societal constructs of perceived difference based on skin color, physical appearance, behavior, etc. When we step back, we can see that this use of “race” actually has no intrinsic biologic meaning, and that it is only my suggested meaning of “race” as a universal definition of the human species that confers any form of intrinsic genetic truth. While this discussion may seem merely semantical, and I agree that there are no inherent positive or negative attributes to either definition of “race,” I would suggest that our societies require more intentional efforts at understanding interconnectedness and universal biological truths than we do at understanding categorical differences. With our cognitive biases of negativity over positivity and categorical organization over universal wholeness, I feel that it is imperative to utilize language that is intentional in its efforts at unification and interconnectedness even if its initial appearance feels semantical.
Race vs. Ethnicity
If “race” is indeed a societal construct with no real biological relevance, are there better terms to describe the uniqueness of various human bio-psycho-social forms? Again this discussion could become semantical rather quickly, so I will simply offer a personal definition that will help shape our future discussion. Instead of using the term “race” to divide humanity into seemingly different physical and biological forms, we can use the useful term and concept of ethnicity to honor ancestral and cultural heritage. Ethnicity in this form can have both biological and cultural meanings, referring to the origins of birth, common languages, cultural practices and even food preferences of individuals. Underneath the large umbrella of ethnicity we can house an expansive worldview that honors the unique origins and development of an individual within a specific cultural or religious tradition, but also acknowledges the universal truths and connections of the one human race. As a physician practicing evolutionary medicine, I see that it is critical to understand the ethnicity and culture of an individual within the context of larger human racial evolution in order to support them optimally. Some of you may be saying to yourself, “This is not how traditional medicine is practiced; isn’t genetics involved in here somewhere?” Indeed, I would be surprised given the common narrative of “race,” health and genetics if you did not have these questions, and I will be the first to say that even I, in my very early stages of medical training, held dogmatic beliefs about genetics that were simply waiting to be dismantled.
An Introduction to the History of Medicine and Genetics
Traditional medicine has embraced the societal construct of “race” and has sought to apply layers of biologic and genetic science to indiscriminately portray the societally constructed concept of “race” as something with actual biological relevance. From 1990 to around 2003, scientists around the world were engrossed in efforts to “sequence” the human genome, to identify the sections of our DNA that provide instructions for making proteins such as enzymes and structural elements. Known as The Human Genome Project, the scientific effort was arguably the greatest collective scientific effort of our time. Before we go any further in this exploration, however, let’s start with some key definitions within the world of genetics.
What Exactly is DNA?
DNA, short for deoxyribonucleic acid, is the functional unit for carrying genetic information in millions of organisms such as humans. Structurally, DNA is formed as an enormously long double stranded helix. Each strand is made up of a sequence of nucleotides that contain three essential components. The first two components of a nucleotide are a specialized sugar and a phosphate group. Together these two components create the backbone or outside of each stand of the helix. If we simply looked at DNA as two chains of sugar and phosphate groups, however, there would be no individual variation, just long chains of sugars and phosphates lacking any real “genetic information.” So what then makes DNA unique to me and other humans? It all comes down to the third component of the nucleotide: the nitrogenous base.
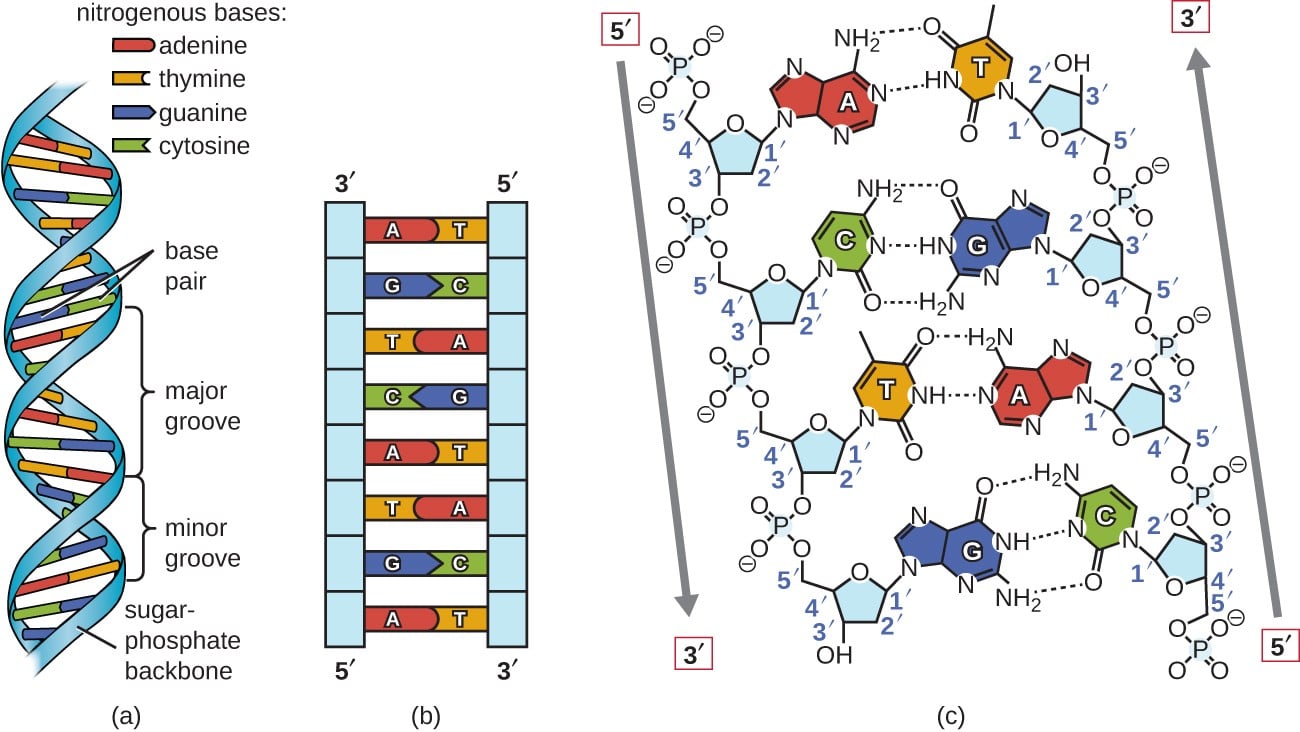
Extending inward from the sugar phosphate backbone, each DNA strand has precisely one nitrogenous base. It would seem reasonable to think that in order to store all the unique information needed to make such complex organisms that there would be hundreds of thousands of nitrogenous bases at different positions in the DNA helix. The reality? There are only 4 nitrogenous bases in human DNA: adenine (A), thymine (T), guanine (G), cytosine (C). Yes, just 4. What perhaps is even more fascinating than the fact that all of the genetic information in humans is communicated with just 4 unique bases, is that functionally the bases act as pairs, creating just 2 functional units! With its double stranded helical structure, DNA is actually a unified molecule with matching elements from each individual strand that ensures its overall structural stability. When we look at the nitrogenous bases on one strand compared to the nitrogenous bases on the other complementary strand, we find that the core of DNA, the interface between the two strands is created by bonds between two different base pairs: either guanine (G) with cytosine (C) or adenine (A) with thymine (T). Looking deeper we see that the reason guanine can only bind with cytosine and not adenine or thymine is that guanine and cytosine are connected via 3 hydrogen bonds while adenine and thymine are connected by just 2. It really is rather profound to think that all the genetic information required to create and maintain the human organism is contained in a self replicating string of nucleotides that can only be uniquely identified by the differences in its sequences of four different nitrogenous bases!
The Human Genome (Confusion) Project
Getting back to our story about The Human Genome Project, you may then be asking, why in the world would it take over 10 years to sequence the human genome? How many of these unique base pairs and genes do humans on average actually have? This question has been debated and debated since the first models of the human genome were being released. To prove just how much these numbers have changed this century, I will share data from two studies. The first study was published in early 2001 and stated “There appear to be about 30,000–40,000 protein-coding genes in the human genome—only about twice as many as in worm or fly.” (1). The second study, published in 2019 reported in Table 1 of their findings that there were precisely 19,116 protein-coding genes in the human genome resulting in “the protein-coding non-redundant transcriptome space containing around 59,281,518 base pairs”, or just under 2% of the entire genome (2). In comparing these two studies and the large discrepancy in their reported number of protein-encoding genes, it is important to point out a few things.
First, simply getting the sequence of base pairs from the genome does not really tell you anything functionally about those sequences. Which sequences of base pairs are actually unique to a particular gene and involved in creating protein products from that gene? The majority of research that has continued from the original sequencing project has really focused on identifying the “functional” genome, how many unique genes do we actually have within all of the base pairs that act as instructions (also known as protein-coding genes) for making various proteins and molecules. In addition, since it is now recognized that approximately 98% of our entire genome is not clearly used to make identifiable protein products, there has been extensive research into how many unique genes may exist that indirectly affect and regulate the protein-coding genes. In the beginning of this functional exploration, it was markedly unclear what these non-coding sections of DNA were actually doing for humans as most of the focus and hypotheses were based on figuring out which sections of the genome told the human form to create proteins, not which sections made regulatory elements. To this date we still do not really know how many protein-coding and non-coding genes we have in the genome, but the best estimates appear to show that we likely have a near equivalent number of non-coding genes to the ~20,000 protein coding genes, putting the total number of unique genes near the 40,000 mark, the original estimate for the protein-coding genes alone in 2000 (3).
In providing this brief introduction into genes and the research behind human genetics, I hope you can see just how dynamic our understanding really is of the human genome. From this understanding of dynamic complexity and relative uncertainty, let’s begin to dismantle some myths about genetic variability and what this may actually represent for human health and disease.
The Truth Behind Genetic Variation
When we start to look at the collective human genome, protein coding, non-coding and additional DNA segments, most research points to a total somewhere around 3 billion base pairs [6 billion bases in the double helix] (4). Understanding that the genetic variation from one individual to the next is determined solely by the unique sequence of these nitrogenous base pairs, how much variation would you expect to see between one individual to the next? 1%? 5%? 10%? While you likely can see given my previous paragraphs than any number I would provide is merely our current best educated guess, the inter-individual variation appears to be around 0.1% (4). Yes, 0.1%. Now it is impossible to jump from this 0.1% estimate of genetic variation from one individual to another and say then that there should only be a 0.1% difference in health or disease outcomes between individuals due to genetic variation alone, but it certainly thickens the discussion behind genetic determinism. For instance, gene variations such as those behind the “sickling” of red blood cells in sickle cell disease are so incredibly minute in the total collective genome, but manifest physically or phenotypically as complex disease that certainly creates differing health outcomes far beyond the mere 0.1% of genetic variation between individuals. That being said, there is still also massive variation in health outcomes in those with sickle cell disease that appears dependent on many lifestyle, psychological and environmental exposures, pointing again to the fact that genes alone, even in a fairly deterministic condition as sickle cell disease, are not destiny (5). As the story deepens and deepens, we have to start asking ourselves some really tough questions:
How much of my health is related strictly to the differences in my genetics compared to others?
How much of my health is related strictly to the differences in my genetics within my family?
Is there a deeper story behind genetics such that the mere presence of certain genetic variations is not actually as important as how certain patterns of genes are actually being expressed?
I could keep going on and on with these questions, but there is something perhaps even more surprising than the inter-individual genetic variability of 0.1% that I would like to share with you now.
Taken from the previously cited NIH resource (4), please read this next section and reflect on the meaning of the author’s words.
“Notwithstanding the genetic differences between individuals, all humans have a great deal of their genetic information in common. These similarities help define us as a species. Furthermore, genetic variation around the world is distributed in a rather continuous manner; there are no sharp, discontinuous boundaries between human population groups. In fact, research results consistently demonstrate that about 85 percent of all human genetic variation exists within human populations, whereas about only 15 percent of variation exists between populations (Figure 4). That is, research reveals that Homo sapiens is one continuously variable, interbreeding species. Ongoing investigation of human genetic variation has even led biologists and physical anthropologists to rethink traditional notions of human racial groups. The amount of genetic variation between these traditional classifications actually falls below the level that taxonomists use to designate subspecies, the taxonomic category for other species that corresponds to the designation of race in Homo sapiens. This finding has caused some biologists to call the validity of race as a biological construct into serious question.”
Summarizing these statements, we see that there is actually greater genetic variation within an ethnic or geographic population than there is outside of it. Genetically, a Caucasian individual with English ancestry living in the Northeast of the US may actually have more genetically in common with a Black individual with West African ancestry living in the Southwest US than either do with individuals with similar ethnicities and geographic heritage. This on the surface does not appear to make any rational sense, but the genetic data is resoundingly clear, the genes between individuals of different groups is usually more similar than the genes between individuals of the same ethnic group. As I hope you can see, I am not the only one critically assessing how societally constructed “race” appears to have no basis in biologic and genetic inquiry.
Health Disparities and Genetics
Even before The Human Genome Project, the common narrative in medicine pointed to genetics as the harbinger of disease. Family member with diabetes? Watch out. In addition to the focus on genetic family history without context for environmental and lifestyle factors, traditional medicine slowly started treating individuals of different ethnic backgrounds and skin color as if they were entirely different organisms. Pointing to “evidence” from research trials that categorized these “racial” and ethnic differences, the reasons for the variations in treatment outcomes and risks for disease between different “racial” groups was seemingly “scientific” and always chalked up to genetics. This narrative, like so many in medicine that have been proven wrong, was established because it seemed to make sense on the surface. You look different than me so this must be due to genetics. You have a different risk for disease than I do and respond differently to medications than me so these differences must be due to genetics. This cognitive narrative is simple and coherent for almost all humans, even for those for whom it is hurting because, well, it’s simple. The problem? It’s entirely untrue.
You may be thinking now, in the light of the evidence I shared showing you the truth behind genetic variation in humans and different ethic groups, how can medical providers still think these differences in various health outcomes are purely genetic? One major issue involves conscious and unconscious bias. While society and individuals appear to cognitively understand rational, conscious biases, there is a vast arena of poorly understood unconscious or implicit biases, cognitive ideas that shape our decision making without ever reaching full consciousness.
Bias in Medicine
In a controversial study conducted in 2015 at my very own medical school, the University of Virginia, researchers found that a non-trivial percentage of medical students or resident physicians incorrectly identified various statements suggesting biologic/physiological differences between individuals of different ethnicities as true (6). Essentially, the study subjects truly thought that there were biological/physiological differences between certain individuals with different ethnicities when there were none (6). Expanding from this, the researchers also found that these individuals (with incorrect biologic assumptions) were also more likely in written case simulations to respond differently to treating individuals of different ethnicities for various painful medical conditions. In almost all cases the net result of their medical choices in these written simulations was to provide inadequate care to the individual of an ethinic minority (6). While indeed this study has many potential issues, it acts as “a canary in the coal mine” that medical providers unwittingly may have incorrect ideas about physiologic differences between individuals of different ethnicities and thus provide inadequate care because of these cognitive biases. Thinking more deeply, it may even seem that the conscious biases and attributions that nearly all health differences between different ethnicities are purely genetic are arguably more benign and rectifiable than the hidden, unconscious biases. Either way, we cannot accept medical providers practicing within these broken paradigms any longer.
The Truth Behind Health Disparities
Now some of you may be thinking, are you suggesting all the biomedical research conducted with categorizations of ethnicity is entirely irrelevant? No, this research broadly speaking likely shows very real effects and differences between various groups, but the causative elements behind these differences and what these differences actually represent are likely entirely different than anything traditional medicine has ever suggested. What if the large differences observed in elevated blood pressure (hypertension) rates observed between Black Americans and Caucasians has nothing to do with pure genetic differences and everything to do with social determinants of health? (7). Could the high risk “Black American group” really just represent any individual of any ethnicity with poor dietary intake due to limited access to food and poverty? Could this group really just represent any individual of any ethnicity suffering significant childhood trauma? Could this group really just represent any individual of any ethnicity who lives in a family that has had successive generations experiencing systemic oppression, fear, violence and poverty? Could the simple fact that your grandmother, your grandmother’s grandmother, or your grandmother’s grandmother’s grandmother lived in a state of oppression, fear and trauma because of institutionalized violence and racism be the reason for the health disparities we are attributing to “racial differences and genetics?” What I have just described to you as the generational effects of trauma and racism is not just a theoretical idea, but a concept rooted in scientific rigor known broadly as “epigenetics.” Let’s explore this more.
Epigenetics
Epigenetics can most simply be described as the field of study seeking to examine all the factors, internal, external and inherited that affect the expression of one’s individual genetics. You see, your genetics, your DNA is not a blueprint from birth. Simple stimuli such as food intake and sun exposure will send signals through the body that literally turn on and off the expression of certain genes and signalling pathways. All of your genes are not being “expressed” at all times. It’s not physiologically compatible with life. With complex regulatory networks and inhibiting pathways, we can’t have competing pathways on at the same time confusing cells as to what they should be doing. When we start to realize that our environment and the environment of our ancestors is literally informing minute to minute how our DNA is being expressed, we can finally be freed from the false world of “genetic determinism.”
In his pioneering book, Regenerate, author Sayer Ji lays out arguably the best introduction to an evolved discussion of genetics and genetic expression that I have read to date (8). Starting with the powerful premise of epigenetics,Ji cites multiple studies, both in humans and other organisms showing the generational effects of toxin exposures, stress, famine, violence and oppression. One of the most widely discussed studies showing the enduring effects of environmental exposures on epigenetics and genetic expression involves offspring from “The Dutch Hunger Winter of 1944-45.” In this groundbreaking study, researchers reported that “These data are the first to contribute empirical support for the hypothesis that early-life environmental conditions can cause epigenetic changes in humans that persist throughout life.” (9).
Now some in the scientific community have felt that the “Dutch Hunger Winter” study, as well as similar research conducted by Rachel Yehuda are stretching the conclusions we can make about transgenerational inheritance through epigenetic mechanisms, citing ideas such as, at least for females, that their ovaries containing germline genetic information are fully developed while in the in-utero environment of the mother (think about that crazy fact just for a second!) and that we would need to study multiple generations of individuals beyond the index generation experiencing trauma, for example, to definitely claim epigenetic mechanisms of inheritance, but these arguments, in my opinion, become unnecessarily semantical, when at the core, what cannot be lost in all of this research is the simple fact that experiences in mothers and grandmothers appear to have enduring effects on the expression of genes in offspring, and studying the genes alone will not provide us with the answers behind differences in health outcomes (9,10).
What Can Nematodes Teach Us About Epigenetics?
While we do not as yet have many large human cohorts followed over many generations to better examine epigenetic mechanisms behind disease, there is a wealth of animal data showing the rather fantastical effects of transgenerational inheritance. Using the well understood nematode known as C. elegans, researchers found something astonishing (11,12). When exposing the worms of one generation to increased temperatures that activated the expression of a heat-shock protein causing the worms to “glow” a reddish color, they noticed that five successive generations following the exposed generation that were not exposed to the high temperatures themselves still showed increased genetic expression in the heat-shock protein and displayed degrees of the reddish color glow noticed in the index generation (12). Examining the potential mechanisms behind this further, the researchers found modifications in the histones (proteins) around DNA during early embryonic development that were affecting genetic expression at an epigenetic level (12). In a second experiment, researchers noted that when five successive generations of worms were exposed to the same higher temperatures, there was an enduring epigenetic effect in worms not exposed to the higher temperature for 14 future generations (12)!
While many are right to pause before broadly extrapolating such findings and the underlying mechanisms of epigenetic inheritance in nematodes to humans, it is important to acknowledge two things. First, C. elegans appears to have at least 40% homology in its genes when compared to humans (13). What does this mean? From a functional perspective, humans and C. elegans appear to share about 40% of their functional genome, even if the exact sequences of nitrogenous bases, for example, in certain genes are slightly different. In addition, when we look to compare organisms based on conserved genetics or genetic homology, we must not leave out potential conserved patterns of epigenetic inheritance, also known as “comparative epigenomics:” Essentially, while certain species may only show certain degrees of homology when looking at specific protein-coding genes, species may actually display very homologous or conserved patterns of epigenetic inheritance, making the mechanisms behind epigenetics more universal/similar and important that the specific genes that are actually involved/expressed (14).
Epigenetic Implications for Humans
Scientists are by nature conservative. The conclusion of nearly every research study ends with “these results are preliminary and more research is required.” Even in my own research I take a cautious approach, but this cannot stop us from curious and thoughtful inquiry. What if the research from C. elegans, the “Dutch Hunger Winter” and other similar studies is trying to tell us something? What if we are slowly awakening to the reality that the effects of racism, oppression and violence against humans across all ethnicities over hundreds of years has been exerting detrimental effects on the lives of humans today within affected generational lineages, even if individuals today are currently living in the absence of slavery (many continue to live with violenc eand poverty). What if to really change the trajectory of ethnic groups historically oppressed and living in states of trauma and fear, we cannot just try to make subtle changes for one generation, but instead, must dismantle destructive institutions and maintain communities of opportunity and abundance for several consecutive generations to come? What if it is reasonable to expect that we could realistically continue to see worse health outcomes in generationally affected ethnic groups for many generations to come via the previously described epigenetic mechanisms, even if we do finally begin to make some of the radical changes necessary to our societies? We have no time to waste!
The questions I am asking here are very real, and while the picture they paint may seem bleak, the reality and opportunity for healing is actually more optimistic than the purely genetic theories behind disease would ever suggest.
Food as Information, Lifestyle Changes Causing Changes in Genetic Expression
It can be fairly easy to look at both genetics and even epigenetics as mechanisms of disease continuation, destinies sealed before you were even conscious of your own being. While I cannot give you a rigorous scientific explanation behind my counter to this ideology, I want to share my perspective on this idea so that it might help frame this exploration. Epigenetics and genetic inheritance, in my view, can best be understood as “potential” or “potential energy.” We all come into this life with varying degrees of genetic and epigenetic “potential” that can be further affected by the environmental exposures we face. Much of life for that matter, in my worldview, can be looked at as dynamically changing degrees of “potential” and expressed energy. While I have been sharing foundational definitions and research around epigenetic expression in this discussion, I have yet to give you a concrete mechanism for how this “potential” can be modified.
Returning to the previously mentioned groundbreaking book Regenerate by Sayer Ji, we are provided with perhaps one of the most monumental discoveries for all life. In the opening chapter of his book, Sayer outlines some of the newest research behind microRNA’s, specialized molecules that act to turn on and off genetic expression downstream of the actual transcription event of DNA. While we are just beginning to understand how human derived microRNA’s interact with messenger RNA (mRNA) to regulate genetic expression in humans, there is an entirely separate field of microRNA study that points to something even more astonishing (15). Researchers have begun to isolate microRNA’s derived from other organisms, such as plants, and have speculated that, given their ability to cross the gastrointestinal tract intact, that these microRNA can actually have regulatory effects on our genetic expression in ways similar to our own endogenous microRNA’s (16,17). Yes, you read this correctly, plants and even animal foods you consume in your diet can have specialized molecules (microRNA’s) that are absorbed into the GI tract into the bloodstream and enter into various cells to modify or regulate human genetic expression. This is in essence universal cross species communication in real time.
In a follow-up from the original AIP and Inflammatory Bowel Disease (IBD) study published in 2017, researchers from Scripps in San Diego demonstrated positive changes in genetic expression across functional domains of immune function and wound healing in intestinal cells taken from participants following the AIP diet and other lifestyle habits. (18). The timeline for the changes in genetic expression? Less than 3 months. Coupled with this emerging understanding of how our food can literally be information for our body across multiple levels including genetic expression, there is a wealth of emerging science showing the amazing potential of diet and lifestyle to positively impact one’s inherited or “genetic” potential in a matter of days to weeks. While I must stress that there, indeed, must be widespread dramatic changes to many of our most integral communal institutions to make the progress needed in improving health outcomes for those historically oppressed and traumatized, there is nothing more empowering than realizing that your genetic inheritance is not destiny and that the food you eat can literally positively change how your genes are being expressed, leading to healing and more optimal health.
Conclusions
We have traversed a lot of ground with this discussion. Beginning with my intentions for writing this piece, I moved into a discussion seeking to define “race” on societal and biologic terms. We explored the differences between race and ethnicity, and discovered that the concept of societally constructed “race” has actually no biological or genetic relevance. From there we explored the human genome and genetics, digging into the components of our DNA to really understand what makes us, us. With this foundation, we then explored the state of our understanding of the human genome and discovered that there is actually greater genetic variation among people within similar populations and ethnic groups than between them, and that overall, inter-individual genetic variability between humans is likely only around 0.1%. Expanding the discussion of genetics into the realms of health and medicine, we began to see how clinical care has actually taken a misguided approach to its understanding of “race” and genetics, with biases, both unconscious and conscious, compromising the delivery of optimal healthcare to individuals of certain ethnic groups.
Moving further, we started to speculate as to the real causative factors behind the many observed differences in health outcomes between different ethnic groups, seeing perhaps, that many of the most commonly understood differences in health and disease risk between different ethinc groups may actually have nothing to do with “race” and everything to do with social determinants of health, lifestyle, generational trauma, and maladaptive, inherited patterns of genetic expression (epigenetics). Delving into the science of epigenetics and pioneering research into the role of environmental exposures and trauma to impact the health of generations far after the actual trauma itself, we started to dismantle the ideas of genetic determinism and see that generations of violence, oppression, famine and poverty can have long lasting effects far after their actual occurrence in time. To close the discussion, we finished with an optimistic exploration of how diet and lifestyle can have fairly immediate positive effects on genetic expression and how plants (food) can actually act as real time information modifying how our genes are expressed. Basking in the reality of our interconnected nature with other forms of life, I provided a final call to action urging dramatic, but sustainable change across our communities and institutions including healthcare in order to begin to shift the trajectory of health for all humans epigenetically inheriting generations of oppression, violence, hatred and fear.
CLICK TO EXPAND // References
References
- Lander, E., Linton, L., Birren, B. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001). https://doi.org/10.1038/35057062
- Piovesan, A., Antonaros, F., Vitale, L. et al. Human protein-coding genes and gene feature statistics in 2019. BMC Res Notes 12, 315 (2019). https://doi.org/10.1186/s13104-019-4343-8
- Salzberg SL. Open questions: How many genes do we have?. BMC Biol. 2018;16(1):94. Published 2018 Aug 20. doi:10.1186/s12915-018-0564-x
- National Institutes of Health (US); Biological Sciences Curriculum Study. NIH Curriculum Supplement Series [Internet]. Bethesda (MD): National Institutes of Health (US); 2007. Understanding Human Genetic Variation. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20363/
- Serjeant GR, Vichinsky E. Variability of homozygous sickle cell disease: The role of alpha and beta globin chain variation and other factors. Blood Cells Mol Dis. 2018;70:66-77. doi:10.1016/j.bcmd.2017.06.004
- https://news.virginia.edu/content/study-links-disparities-pain-management-racial-bias
- Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2):135-138. doi:10.1097/MAJ.0000000000000308
- Ji, S. (2020). Regenerate: Unlocking your body's radical resilience through the new biology. Carlsbad, CA: Hay House.
- Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046-17049. doi:10.1073/pnas.080656010
- Yehuda R, Lehrner A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry. 2018;17(3):243-257. doi:10.1002/wps.20568
- A. Klosin et al. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320-23.
- Weinhouse C, Truong L, Meyer JN, Allard P. Caenorhabditis elegans as an emerging model system in environmental epigenetics. Environ Mol Mutagen. 2018;59(7):560-575. doi:10.1002/em.22203
- Sin O, Michels H, Nollen EA. Genetic screens in Caenorhabditis elegans models for neurodegenerative diseases. Biochim Biophys Acta. 2014;1842(10):1951-1959. doi:10.1016/j.bbadis.2014.01.015
- Lowdon RF, Jang HS, Wang T. Evolution of Epigenetic Regulation in Vertebrate Genomes. Trends Genet. 2016;32(5):269-283. doi:10.1016/j.tig.2016.03.001
- Plotnikova O, Baranova A, Skoblov M. Comprehensive Analysis of Human microRNA-mRNA Interactome. Front Genet. 2019;10:933. Published 2019 Oct 8. doi:10.3389/fgene.2019.00933
- Zhang H, Li Y, Liu Y, et al. Role of plant MicroRNA in cross-species regulatory networks of humans. BMC Syst Biol. 2016;10(1):60. Published 2016 Aug 8. doi:10.1186/s12918-016-0292-1
- Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? [published correction appears in Nutr Metab (Lond). 2018 Oct 18;15:74]. Nutr Metab (Lond). 2018;15:68. Published 2018 Oct 1. doi:10.1186/s12986-018-0305-8
- Chandrasekaran A, Molparia B, Akhtar E, et al. The Autoimmune Protocol Diet Modifies Intestinal RNA Expression in Inflammatory Bowel Disease. Crohns Colitis 360. 2019;1(3):otz016. doi:10.1093/crocol/otz016
















0 comments